LG Chem promises to permanently save consumers from overheating problems of lithium batteries. The graphite-based composite material developed by the company has the ability to change its molecular structure when heated, making it completely impossible for batteries to ignite in the event of a short circuit. This will change the world of batteries, the company is confident.
Traditionally, the cathodes and anodes of batteries are covered with copper or aluminum foil to draw current from the electrodes. South Korean scientists have set out to develop a non-metallic conductor foil that acts as an insulator when overheating. This would disable the cell and prevent the effects of short circuits - the main cause of overheating and fire in lithium batteries.
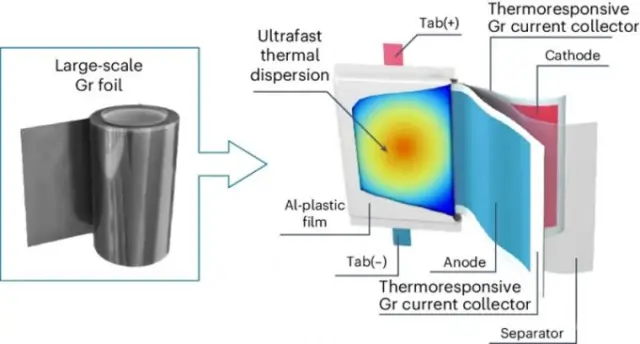
During the scientific research, the researchers created a composite SRL (Safety Reinforced Layer) with inclusions of graphite (LiNi0.8Co0.1Mn0.1O2||graphite), which when heated above a critical level for the battery increases its resistance. The material demonstrated the ability to increase resistance by 5,000 ohms when heated for every degree Celsius. This breaks the fatal chain leading to battery ignition, as demonstrated by LG Chem.
The proposed composite films, only one micron thick, are also an order of magnitude better at conducting heat than aluminum or copper foil. This helps the battery respond more quickly to heat and can protect it from overheating. The composite material replaces the metal foil as a conductor, and its production is optimized for roll production. At the same time, LG Chem is not yet ready for mass production of the new product and promises to continue improving this technology, also intending to demonstrate non-flammable car batteries based on it as early as 2025.
